EIQ updates on Cat III code application
Our AI heart disease detection Investment EchoIQ (ASX: EIQ) put out an update on its application for a category III reimbursement code for its Aortic Stenosis tech yesterday.
Unfortunately, EIQ’s application was unsuccessful for the second time this year - which explains the sell down yesterday to a low of ~17.5c per share.
EIQ recovered today with some buying back up to 18c at the time of us writing this.
One thing we noticed in yesterday’s announcement that may have been a trigger for the recovery today was EIQ’s discussions around a new AI-specific coding framework (Clinically Meaningful Algorithmic Analysis) that is being developed by the AMA.
(The AMA is the body that approves/recommends reimbursement codes).
EIQ said that during the last round of meetings with the CPT editorial panel on the 18th of September the board was considering whole new reimbursement codes for AI tech which is “anticipated to be implemented during CY2026”.
Basically, it read to us like EIQ’s tech would be in a far stronger position for approvals next year once that new framework had been set up.
Unfortunately, in the short term that will mean EIQ needs to work with customers on utilising the Misc code (93799) that its tech is currently operating under.
The misc code means the company can continue to bill for usage, however claims may require additional documentation and administrative burden so payments may be more uncertain due to lower approval rates from insurance.
Cat III would have meant higher reimbursement rates and a pathway to the holy grail cat I code.
We will now have to wait until next year for the Aortic Stenosis detection tech.
In the meantime the focus will still be on commercialisation for the Aortic Stenosis tech AND getting the company’s Heart Failure detection tech FDA ready…
EIQ is going for FDA approvals for its Heart Failure detection tech
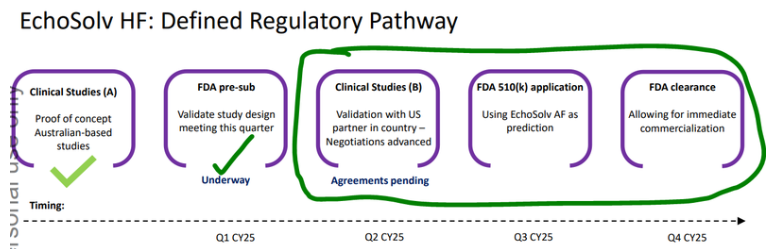
EIQ is also developing its technology for a second heart condition: Heart Failure.
The Heat Failure tech is going through a clinical validation study with the illustrious May Clinic right now, and FDA clearance is expected before the end of the year.
The Heart Failure FDA clearance will be another major catalyst for EIQ for two reasons:
- Because Heart Failure is a much bigger market to Aortic Stenosis: Healthcare expenditure for AS is ~US$10BN per annum, expenditure for Heart Failure is ~US$70BN.
- Because the Heart Failure tech already has an established reimbursement code pathway in place so EIQ can switch on revenues a lot quicker...
Before the end of the year could have FDA approvals for its Heart Failure AI tech.
The big win then for EIQ will be that the Heart Failure tech will benefit from all the rollout work EIQ is doing on Aortic Stenosis.
Because EIQ’s is a software product - it is very much “plug and play”.
Any hospital networks that are already using EIQ for Aortic Stenosis, can easily set up for Heart Failure as well... once the FDA clearance comes in which EIQ is aiming for by the end of the year.
(Source)
What’s next for EIQ?
🔄 Commercialisation updates for Aortic Stenosis AI tech
The key metric we will be tracking in the short term is how many integrations EIQ can secure for its Aortic Stenosis tech.
In the short term we want to see more distribution deals - either through strategic partnerships or reseller deals.
🔄 Heart Failure clinical validation study
We want to see EIQ complete these trials and (hopefully) deliver strong enough results to support an FDA submission for its Heart Failure detection tech.
🔄 Australia and NZ pilot program
EIQ has previously mentioned that this program is being run with a ”leading global structural heart innovation company”.
We want to see some more news on this front because we think it could help advance EIQ’s licensing revenue pathway and be a “proof of concept” study that EIQ can take into the US.
🔄 Partnership with European reseller to broaden market exposure
We want to see EIQ expand into new markets like Europe, in a previous webinar EIQ said the company was pursuing this opportunity and last week the company updated that there was progress on this with negotiations underway.
This also includes CE Mark and TGA applications so that EIQ can sell into Europe and Australia.






